How Do Fuel Cells Work?
Fuel cells use hydrogen and oxygen, the molecules that create water, to produce electricity with no pollution. First conceived in 1839, fuel cells are silent electron factories with no moving parts and no combustion. Since that time, companies around the world have been developing and refining the technology as a means of replacing traditional battery and generator technologies and to help address some of the world’s most difficult energy and environmental challenges.
Fuel cells have two adjacent chambers the anode side and the cathode side-separated by a membrane. Hydrogen gas enters the anode side where the atoms react with a platinum catalyst and release electrons. That chamber becomes flooded with free electrons and hydrogen protons, or hydrogen atoms stripped of their electrons.
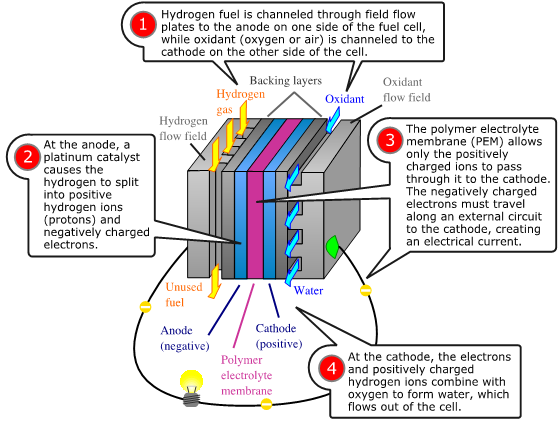
The positively charged hydrogen protons pass through the membrane into the cathode side of the fuel cell. The electrons flow out of the anode side to power a load. After running through the system wiring, the electrons re-enter the fuel cell on the cathode side, completing the electrical path.
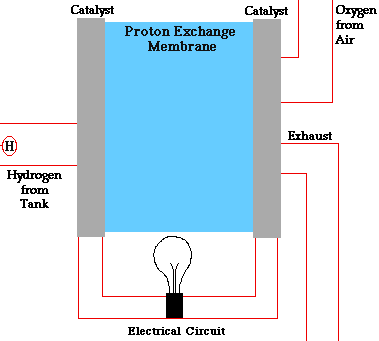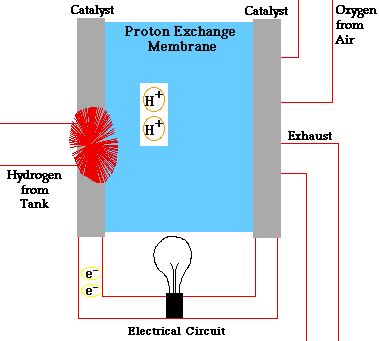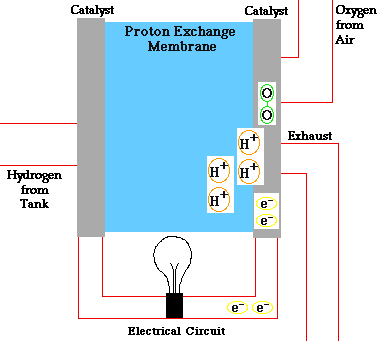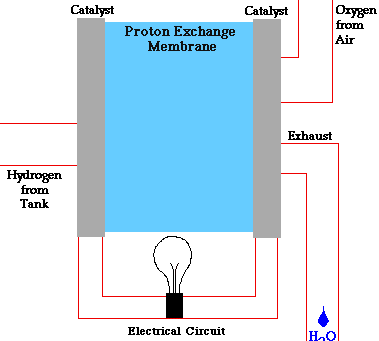
On the cathode side, the hydrogen protons that slipped through the membrane combine with the free electrons and with oxygen molecules from the ambient air to produce pure water. You can envision a fuel cell as a system that borrows electrons from hydrogen, ships them off to do some useful work, such as powering radios, and then grabs them back and partners them with oxygen to form water.
The chemical equation is about as simple as it gets: 2H2 + O2 = 2H2O.
Because fuel cells do not have moving parts and do not rely on combustion, they are easy to maintain, very efficient, and quiet.
PEM Fuel Cell Technology (PEMFC)
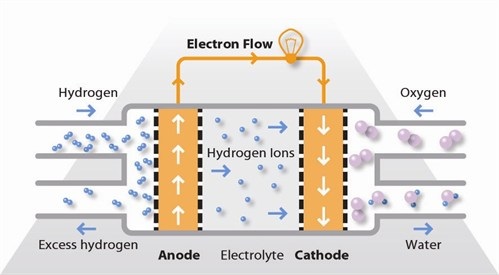
The proton exchange membrane fuel cell (PEMFC) uses a water-based, acidic polymer membrane as its electrolyte, with platinum-based electrodes. PEMFC cells operate at relatively low temperatures (below 100 degrees Celsius) and can tailor electrical output to meet dynamic power requirements. Due to the relatively low temperatures and the use of precious metal-based electrodes, these cells must operate on hydrogen which has 99.99% purity, which is generally available in gas form from a local gas supplier, via electrolysis or reforming.
PEMFC cells are currently the leading technology for light duty vehicles and materials handling vehicles, and for stationary backup and prime power applications. The PEMFC fuel cell is also sometimes called a polymer electrolyte membrane fuel cell.
Hydrogen fuel is processed at the anode where electrons are separated from protons on the surface of a platinum-based catalyst. The protons pass through the membrane to the cathode side of the cell while the electrons travel in an external circuit, generating the electrical output of the cell. On the cathode side, another precious metal electrode combines the protons and electrons with oxygen to produce water, which is expelled as the only waste product; oxygen can be provided in a purified form, or extracted at the electrode directly from the air.
High Temperature PEM Fuel Cell (HT PEMFC)
A variant of the PEMFC, which operates at elevated temperatures is known as the high temperature PEMFC (HT PEMFC). By changing the electrolyte from being water-based to a mineral acid-based system, HT PEMFCs can operate up to 200 degrees Celsius. If the fuel source is not hydrogen gas, then the HT PEMFC may overcome some issues with hydrogen fuel purity as HT PEMFC’s are able to process reformate containing small quantities of Carbon Monoxide (CO). The balance of plant can also be simplified by removing the need for a purifier as these systems run at a much higher temperature.
HT PEMFCs are not superior to low temperature PEMFCs; both technologies find niches in where their benefits are preferable. The table below summarises differences between the two PEMFC designs:
| |
Low Temp. PEMFC |
High Temp. PEMFC |
| Operating temperature |
80-100 degrees C |
Up to 200 degrees C |
| Electrolyte |
Water-based |
Mineral acid-based |
| Pt loading
CO tolerance |
0.2-0.8 mg/cm2
<50 parts per million |
1.0-2.0 mg/cm2
1 – 5 % by Volume |
| Impurity tolerance |
99.99% H2 |
Higher |
| Power density |
Higher |
Lower |
| Standby to full load |
3 minutes |
40 minutes |
| Water Management |
Not needed |
Complex |
Direct Methanol Fuel Cell (DFMC)
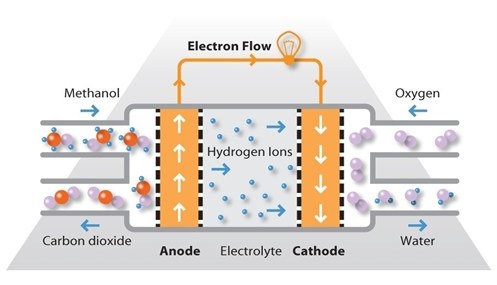
The direct methanol fuel cell (DMFC) is a relatively recent addition to the market. It is similar to the PEM cell, in that it uses a polymer membrane as an electrolyte. However, the platinum-ruthenium catalyst on the DMFC anode can draw the hydrogen from liquid methanol, eliminating the need for a fuel reformer. In this case, pure methanol can be used as fuel, hence the name.
Methanol offers several advantages as a fuel, or indeed as a carrier of hydrogen molecules for use by a fuel cell later. It is inexpensive but has a relatively high energy density and can be easily transported and stored. It can be supplied to the fuel cell unit from a liquid reservoir which can be kept topped up, or in cartridges which can be quickly changed out when spent.
DMFCs operate in the temperature range from 60ºC to 130ºC and tend to be used in applications with modest power requirements, such as mobile electronic devices or chargers and portable power packs. One application for DMFCs which is seeing commercial traction in various countries is the use of DMFC power units for continuously powering cameras or remote monitoring stations with modest loads. These systems seem to have a ‘glass ceiling’ in terms of power output and as such the loads being powered by these systems are generally in the range of 100W to 500W.
Solid Oxide Fuel Cell (SOFC)
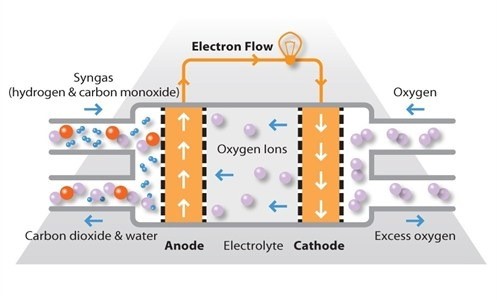
Solid oxide fuel cells work at very high temperatures, the highest of all the fuel cell types at around 800ºC to 1,000°C. They can have efficiencies of over 60% when converting fuel to electricity; if the heat they produced is also harnessed; their overall efficiency in converting fuel to energy can be over 80%.
SOFCs use a solid ceramic electrolyte, such as zirconium oxide stabilised with yttrium oxide, instead of a liquid or membrane. Their high operating temperature means that fuels can be reformed within the fuel cell itself, eliminating the need for external reforming and allowing the units to be used with a variety of hydrocarbon fuels. They are also relatively resistant to small quantities of sulphur in the fuel, compared to other types of fuel cell, and can hence be used with coal gas.
A further advantage of the high operating temperature is that the reaction kinetics are improved, removing the need for a metal catalyst. There are however some disadvantages to the high temperature: these cells take longer to start up and reach operating temperature, they must be constructed of robust, heat-resistant materials, and they must be shielded to prevent heat loss.
There are three different SOFC geometries of SOFC: planar, coplanar and micro-tubular. In the planar design, components are assembled in flat stacks where the air and hydrogen traditionally flow though the unit via channels built in to the anode and cathode. In the tubular design, air is supplied to the inside of an extended solid oxide tube (which is sealed at one end) while fuel flows round the outside of the tube. The tube itself forms the cathode and the cell components are constructed in layers around the tube.
SOFCs are used extensively in large and small stationary power generation: planar types find application in, for example, Bloom Energy’s 100 kW off-grid power generators and SOFCs with output of a few kilowatts are being tested for smaller cogeneration applications, such as domestic combined heat and power (CHP). Micro-tubular SOFCs with output in the watt range are also being developed for small portable chargers.
Phosphoric acid fuel cells (PAFC)
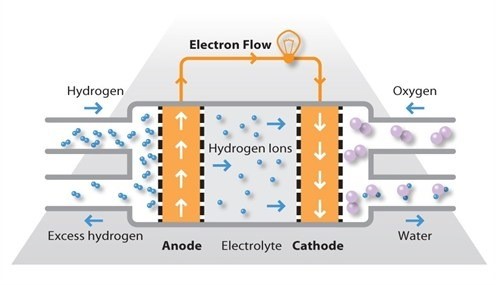
Phosphoric acid fuel cells (PAFCs) consist of an anode and a cathode made of a finely dispersed platinum catalyst on carbon and a silicon carbide structure that holds the phosphoric acid electrolyte. They are quite resistant to poisoning by carbon monoxide but tend to have lower efficiency than other fuel cell types in producing electricity. However, these cells operate at moderately high temperatures of around 180ºC and overall efficiency can be over 80% if this process heat is harnessed for cogeneration.
This type of fuel cell is used in stationary power generators with output in the 100 kW to 400 kW range to power many commercial premises around the world, and they are also finding application in large vehicles such as buses. Most fuel cell units sold before 2001 used PAFC technology.
Molten carbonate fuel cells (MCFC)
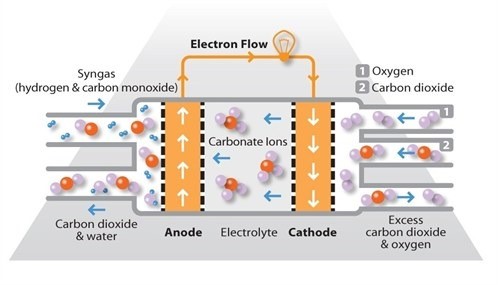
Molten carbonate fuel cells (MCFCs) use a molten carbonate salt suspended in a porous ceramic matrix as the electrolyte. Salts commonly used include lithium carbonate, potassium carbonate and sodium carbonate.
They operate at high temperature, around 650ºC and there are several advantages associated with this. Firstly, the high operating temperature dramatically improves reaction kinetics and thus it is not necessary to boost these with a noble metal catalyst. The higher temperature also makes the cell less prone to carbon monoxide poisoning than lower temperature systems. As a result, MCFC systems can operate on a variety of different fuels, including coal-derived fuel gas, methane or natural gas, eliminating the need for external reformers.
Disadvantages associated with MCFC units arise from using a liquid electrolyte rather than a solid and the requirement to inject carbon dioxide at the cathode as carbonate ions are consumed in reactions occurring at the anode. There have also been some issues with high temperature corrosion and the corrosive nature of the electrolyte but these can now be controlled to achieve a practical lifetime.
MCFCs are used in large stationary power generation. Most fuel cell power plants of megawatt capacity use MCFCs, as do large combined heat and power (CHP) and combined cooling and power (CCP) plants. These fuel cells can work at up to 60% efficiency for fuel to electricity conversion, and overall efficiencies can be over 80% in CHP or CCP applications where the process heat is also utilised.
A German company
MTU Friedrichshafen presented an MCFC at the
Hannover Fair in 2006. The unit weighs 2 tonnes and can produce 240 kW of electric power from various gaseous fuels, including biogas. If fueled by fuels that contain carbon such as natural gas, the exhaust will contain CO2 but will be reduced by up to 50% compared to diesel engines running on marine bunker fuel. The exhaust temperature is 400 °C, hot enough to be used for many industrial processes. Another possibility is to make more electric power via a
steam turbine. Depending on feed gas type, the electric efficiency is between 12% and 19%. A steam turbine can increase the efficiency by up to 24%. The unit can be used for
cogeneration.
Alkaline fuel cells (AFC)
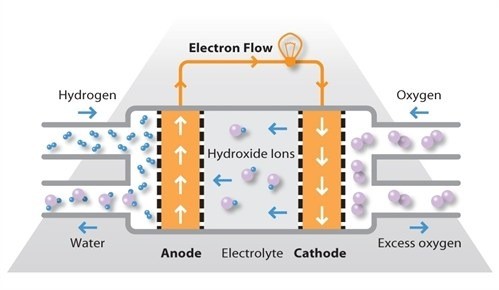
Alkaline fuel cells (AFCs) were one of the first fuel cell technologies to be developed and were originally used by NASA in the space programme to produce both electricity and water aboard spacecraft. AFCs continued to be used on NASA space shuttles throughout the programme, alongside a limited number of commercial applications.
AFCs use an alkaline electrolyte such as potassium hydroxide in water and are generally fuelled with pure hydrogen. The first AFCs operated at between 100ºC and 250ºC but typical operating temperatures are now around 70ºC. Because of the low operating temperature, it is not necessary to employ a platinum catalyst in the system and instead, a variety of non-precious metals can be used as catalysts to speed up the reactions occurring at the anode and cathode. Nickel is the most commonly used catalyst in AFC units.
Due to the rate at which the chemical reactions take place these cells offer relatively high fuel to electricity conversion efficiencies, as high as 60% in some applications. AFCs are the cheapest of fuel cells to manufacture. The catalyst required for the electrodes can be any number of different chemicals that are inexpensive compared to those required for other types of fuel cells.
The commercial prospects for AFCs lie largely with the recently developed bi-polar plate version of this technology, considerably superior in performance to earlier mono-plate versions.
Another recent development is the solid-state alkaline fuel cell, utilizing
alkali anion exchange membranes rather than a liquid. This resolves the problem of poisoning and allows the development of alkaline fuel cells capable of running on safer hydrogen-rich carriers such as liquid urea solutions or metal amine complexes.
The world’s first Fuel Cell Ship
HYDRA used an AFC system with 5 kW net output.
 The positively charged hydrogen protons pass through the membrane into the cathode side of the fuel cell. The electrons flow out of the anode side to power a load. After running through the system wiring, the electrons re-enter the fuel cell on the cathode side, completing the electrical path.
The positively charged hydrogen protons pass through the membrane into the cathode side of the fuel cell. The electrons flow out of the anode side to power a load. After running through the system wiring, the electrons re-enter the fuel cell on the cathode side, completing the electrical path.
 On the cathode side, the hydrogen protons that slipped through the membrane combine with the free electrons and with oxygen molecules from the ambient air to produce pure water. You can envision a fuel cell as a system that borrows electrons from hydrogen, ships them off to do some useful work, such as powering radios, and then grabs them back and partners them with oxygen to form water.
The chemical equation is about as simple as it gets: 2H2 + O2 = 2H2O.
Because fuel cells do not have moving parts and do not rely on combustion, they are easy to maintain, very efficient, and quiet.
On the cathode side, the hydrogen protons that slipped through the membrane combine with the free electrons and with oxygen molecules from the ambient air to produce pure water. You can envision a fuel cell as a system that borrows electrons from hydrogen, ships them off to do some useful work, such as powering radios, and then grabs them back and partners them with oxygen to form water.
The chemical equation is about as simple as it gets: 2H2 + O2 = 2H2O.
Because fuel cells do not have moving parts and do not rely on combustion, they are easy to maintain, very efficient, and quiet.
 The proton exchange membrane fuel cell (PEMFC) uses a water-based, acidic polymer membrane as its electrolyte, with platinum-based electrodes. PEMFC cells operate at relatively low temperatures (below 100 degrees Celsius) and can tailor electrical output to meet dynamic power requirements. Due to the relatively low temperatures and the use of precious metal-based electrodes, these cells must operate on hydrogen which has 99.99% purity, which is generally available in gas form from a local gas supplier, via electrolysis or reforming.
PEMFC cells are currently the leading technology for light duty vehicles and materials handling vehicles, and for stationary backup and prime power applications. The PEMFC fuel cell is also sometimes called a polymer electrolyte membrane fuel cell.
Hydrogen fuel is processed at the anode where electrons are separated from protons on the surface of a platinum-based catalyst. The protons pass through the membrane to the cathode side of the cell while the electrons travel in an external circuit, generating the electrical output of the cell. On the cathode side, another precious metal electrode combines the protons and electrons with oxygen to produce water, which is expelled as the only waste product; oxygen can be provided in a purified form, or extracted at the electrode directly from the air.
The proton exchange membrane fuel cell (PEMFC) uses a water-based, acidic polymer membrane as its electrolyte, with platinum-based electrodes. PEMFC cells operate at relatively low temperatures (below 100 degrees Celsius) and can tailor electrical output to meet dynamic power requirements. Due to the relatively low temperatures and the use of precious metal-based electrodes, these cells must operate on hydrogen which has 99.99% purity, which is generally available in gas form from a local gas supplier, via electrolysis or reforming.
PEMFC cells are currently the leading technology for light duty vehicles and materials handling vehicles, and for stationary backup and prime power applications. The PEMFC fuel cell is also sometimes called a polymer electrolyte membrane fuel cell.
Hydrogen fuel is processed at the anode where electrons are separated from protons on the surface of a platinum-based catalyst. The protons pass through the membrane to the cathode side of the cell while the electrons travel in an external circuit, generating the electrical output of the cell. On the cathode side, another precious metal electrode combines the protons and electrons with oxygen to produce water, which is expelled as the only waste product; oxygen can be provided in a purified form, or extracted at the electrode directly from the air.
 The direct methanol fuel cell (DMFC) is a relatively recent addition to the market. It is similar to the PEM cell, in that it uses a polymer membrane as an electrolyte. However, the platinum-ruthenium catalyst on the DMFC anode can draw the hydrogen from liquid methanol, eliminating the need for a fuel reformer. In this case, pure methanol can be used as fuel, hence the name.
Methanol offers several advantages as a fuel, or indeed as a carrier of hydrogen molecules for use by a fuel cell later. It is inexpensive but has a relatively high energy density and can be easily transported and stored. It can be supplied to the fuel cell unit from a liquid reservoir which can be kept topped up, or in cartridges which can be quickly changed out when spent.
DMFCs operate in the temperature range from 60ºC to 130ºC and tend to be used in applications with modest power requirements, such as mobile electronic devices or chargers and portable power packs. One application for DMFCs which is seeing commercial traction in various countries is the use of DMFC power units for continuously powering cameras or remote monitoring stations with modest loads. These systems seem to have a ‘glass ceiling’ in terms of power output and as such the loads being powered by these systems are generally in the range of 100W to 500W.
The direct methanol fuel cell (DMFC) is a relatively recent addition to the market. It is similar to the PEM cell, in that it uses a polymer membrane as an electrolyte. However, the platinum-ruthenium catalyst on the DMFC anode can draw the hydrogen from liquid methanol, eliminating the need for a fuel reformer. In this case, pure methanol can be used as fuel, hence the name.
Methanol offers several advantages as a fuel, or indeed as a carrier of hydrogen molecules for use by a fuel cell later. It is inexpensive but has a relatively high energy density and can be easily transported and stored. It can be supplied to the fuel cell unit from a liquid reservoir which can be kept topped up, or in cartridges which can be quickly changed out when spent.
DMFCs operate in the temperature range from 60ºC to 130ºC and tend to be used in applications with modest power requirements, such as mobile electronic devices or chargers and portable power packs. One application for DMFCs which is seeing commercial traction in various countries is the use of DMFC power units for continuously powering cameras or remote monitoring stations with modest loads. These systems seem to have a ‘glass ceiling’ in terms of power output and as such the loads being powered by these systems are generally in the range of 100W to 500W.
 Solid oxide fuel cells work at very high temperatures, the highest of all the fuel cell types at around 800ºC to 1,000°C. They can have efficiencies of over 60% when converting fuel to electricity; if the heat they produced is also harnessed; their overall efficiency in converting fuel to energy can be over 80%.
SOFCs use a solid ceramic electrolyte, such as zirconium oxide stabilised with yttrium oxide, instead of a liquid or membrane. Their high operating temperature means that fuels can be reformed within the fuel cell itself, eliminating the need for external reforming and allowing the units to be used with a variety of hydrocarbon fuels. They are also relatively resistant to small quantities of sulphur in the fuel, compared to other types of fuel cell, and can hence be used with coal gas.
A further advantage of the high operating temperature is that the reaction kinetics are improved, removing the need for a metal catalyst. There are however some disadvantages to the high temperature: these cells take longer to start up and reach operating temperature, they must be constructed of robust, heat-resistant materials, and they must be shielded to prevent heat loss.
There are three different SOFC geometries of SOFC: planar, coplanar and micro-tubular. In the planar design, components are assembled in flat stacks where the air and hydrogen traditionally flow though the unit via channels built in to the anode and cathode. In the tubular design, air is supplied to the inside of an extended solid oxide tube (which is sealed at one end) while fuel flows round the outside of the tube. The tube itself forms the cathode and the cell components are constructed in layers around the tube.
SOFCs are used extensively in large and small stationary power generation: planar types find application in, for example, Bloom Energy’s 100 kW off-grid power generators and SOFCs with output of a few kilowatts are being tested for smaller cogeneration applications, such as domestic combined heat and power (CHP). Micro-tubular SOFCs with output in the watt range are also being developed for small portable chargers.
Solid oxide fuel cells work at very high temperatures, the highest of all the fuel cell types at around 800ºC to 1,000°C. They can have efficiencies of over 60% when converting fuel to electricity; if the heat they produced is also harnessed; their overall efficiency in converting fuel to energy can be over 80%.
SOFCs use a solid ceramic electrolyte, such as zirconium oxide stabilised with yttrium oxide, instead of a liquid or membrane. Their high operating temperature means that fuels can be reformed within the fuel cell itself, eliminating the need for external reforming and allowing the units to be used with a variety of hydrocarbon fuels. They are also relatively resistant to small quantities of sulphur in the fuel, compared to other types of fuel cell, and can hence be used with coal gas.
A further advantage of the high operating temperature is that the reaction kinetics are improved, removing the need for a metal catalyst. There are however some disadvantages to the high temperature: these cells take longer to start up and reach operating temperature, they must be constructed of robust, heat-resistant materials, and they must be shielded to prevent heat loss.
There are three different SOFC geometries of SOFC: planar, coplanar and micro-tubular. In the planar design, components are assembled in flat stacks where the air and hydrogen traditionally flow though the unit via channels built in to the anode and cathode. In the tubular design, air is supplied to the inside of an extended solid oxide tube (which is sealed at one end) while fuel flows round the outside of the tube. The tube itself forms the cathode and the cell components are constructed in layers around the tube.
SOFCs are used extensively in large and small stationary power generation: planar types find application in, for example, Bloom Energy’s 100 kW off-grid power generators and SOFCs with output of a few kilowatts are being tested for smaller cogeneration applications, such as domestic combined heat and power (CHP). Micro-tubular SOFCs with output in the watt range are also being developed for small portable chargers.
 Phosphoric acid fuel cells (PAFCs) consist of an anode and a cathode made of a finely dispersed platinum catalyst on carbon and a silicon carbide structure that holds the phosphoric acid electrolyte. They are quite resistant to poisoning by carbon monoxide but tend to have lower efficiency than other fuel cell types in producing electricity. However, these cells operate at moderately high temperatures of around 180ºC and overall efficiency can be over 80% if this process heat is harnessed for cogeneration.
This type of fuel cell is used in stationary power generators with output in the 100 kW to 400 kW range to power many commercial premises around the world, and they are also finding application in large vehicles such as buses. Most fuel cell units sold before 2001 used PAFC technology.
Phosphoric acid fuel cells (PAFCs) consist of an anode and a cathode made of a finely dispersed platinum catalyst on carbon and a silicon carbide structure that holds the phosphoric acid electrolyte. They are quite resistant to poisoning by carbon monoxide but tend to have lower efficiency than other fuel cell types in producing electricity. However, these cells operate at moderately high temperatures of around 180ºC and overall efficiency can be over 80% if this process heat is harnessed for cogeneration.
This type of fuel cell is used in stationary power generators with output in the 100 kW to 400 kW range to power many commercial premises around the world, and they are also finding application in large vehicles such as buses. Most fuel cell units sold before 2001 used PAFC technology.
 Molten carbonate fuel cells (MCFCs) use a molten carbonate salt suspended in a porous ceramic matrix as the electrolyte. Salts commonly used include lithium carbonate, potassium carbonate and sodium carbonate.
They operate at high temperature, around 650ºC and there are several advantages associated with this. Firstly, the high operating temperature dramatically improves reaction kinetics and thus it is not necessary to boost these with a noble metal catalyst. The higher temperature also makes the cell less prone to carbon monoxide poisoning than lower temperature systems. As a result, MCFC systems can operate on a variety of different fuels, including coal-derived fuel gas, methane or natural gas, eliminating the need for external reformers.
Disadvantages associated with MCFC units arise from using a liquid electrolyte rather than a solid and the requirement to inject carbon dioxide at the cathode as carbonate ions are consumed in reactions occurring at the anode. There have also been some issues with high temperature corrosion and the corrosive nature of the electrolyte but these can now be controlled to achieve a practical lifetime.
MCFCs are used in large stationary power generation. Most fuel cell power plants of megawatt capacity use MCFCs, as do large combined heat and power (CHP) and combined cooling and power (CCP) plants. These fuel cells can work at up to 60% efficiency for fuel to electricity conversion, and overall efficiencies can be over 80% in CHP or CCP applications where the process heat is also utilised.
A German company MTU Friedrichshafen presented an MCFC at the Hannover Fair in 2006. The unit weighs 2 tonnes and can produce 240 kW of electric power from various gaseous fuels, including biogas. If fueled by fuels that contain carbon such as natural gas, the exhaust will contain CO2 but will be reduced by up to 50% compared to diesel engines running on marine bunker fuel. The exhaust temperature is 400 °C, hot enough to be used for many industrial processes. Another possibility is to make more electric power via a steam turbine. Depending on feed gas type, the electric efficiency is between 12% and 19%. A steam turbine can increase the efficiency by up to 24%. The unit can be used for cogeneration.
Molten carbonate fuel cells (MCFCs) use a molten carbonate salt suspended in a porous ceramic matrix as the electrolyte. Salts commonly used include lithium carbonate, potassium carbonate and sodium carbonate.
They operate at high temperature, around 650ºC and there are several advantages associated with this. Firstly, the high operating temperature dramatically improves reaction kinetics and thus it is not necessary to boost these with a noble metal catalyst. The higher temperature also makes the cell less prone to carbon monoxide poisoning than lower temperature systems. As a result, MCFC systems can operate on a variety of different fuels, including coal-derived fuel gas, methane or natural gas, eliminating the need for external reformers.
Disadvantages associated with MCFC units arise from using a liquid electrolyte rather than a solid and the requirement to inject carbon dioxide at the cathode as carbonate ions are consumed in reactions occurring at the anode. There have also been some issues with high temperature corrosion and the corrosive nature of the electrolyte but these can now be controlled to achieve a practical lifetime.
MCFCs are used in large stationary power generation. Most fuel cell power plants of megawatt capacity use MCFCs, as do large combined heat and power (CHP) and combined cooling and power (CCP) plants. These fuel cells can work at up to 60% efficiency for fuel to electricity conversion, and overall efficiencies can be over 80% in CHP or CCP applications where the process heat is also utilised.
A German company MTU Friedrichshafen presented an MCFC at the Hannover Fair in 2006. The unit weighs 2 tonnes and can produce 240 kW of electric power from various gaseous fuels, including biogas. If fueled by fuels that contain carbon such as natural gas, the exhaust will contain CO2 but will be reduced by up to 50% compared to diesel engines running on marine bunker fuel. The exhaust temperature is 400 °C, hot enough to be used for many industrial processes. Another possibility is to make more electric power via a steam turbine. Depending on feed gas type, the electric efficiency is between 12% and 19%. A steam turbine can increase the efficiency by up to 24%. The unit can be used for cogeneration.
 Alkaline fuel cells (AFCs) were one of the first fuel cell technologies to be developed and were originally used by NASA in the space programme to produce both electricity and water aboard spacecraft. AFCs continued to be used on NASA space shuttles throughout the programme, alongside a limited number of commercial applications.
AFCs use an alkaline electrolyte such as potassium hydroxide in water and are generally fuelled with pure hydrogen. The first AFCs operated at between 100ºC and 250ºC but typical operating temperatures are now around 70ºC. Because of the low operating temperature, it is not necessary to employ a platinum catalyst in the system and instead, a variety of non-precious metals can be used as catalysts to speed up the reactions occurring at the anode and cathode. Nickel is the most commonly used catalyst in AFC units.
Due to the rate at which the chemical reactions take place these cells offer relatively high fuel to electricity conversion efficiencies, as high as 60% in some applications. AFCs are the cheapest of fuel cells to manufacture. The catalyst required for the electrodes can be any number of different chemicals that are inexpensive compared to those required for other types of fuel cells.
The commercial prospects for AFCs lie largely with the recently developed bi-polar plate version of this technology, considerably superior in performance to earlier mono-plate versions.
Another recent development is the solid-state alkaline fuel cell, utilizing alkali anion exchange membranes rather than a liquid. This resolves the problem of poisoning and allows the development of alkaline fuel cells capable of running on safer hydrogen-rich carriers such as liquid urea solutions or metal amine complexes.
The world’s first Fuel Cell Ship HYDRA used an AFC system with 5 kW net output.
Alkaline fuel cells (AFCs) were one of the first fuel cell technologies to be developed and were originally used by NASA in the space programme to produce both electricity and water aboard spacecraft. AFCs continued to be used on NASA space shuttles throughout the programme, alongside a limited number of commercial applications.
AFCs use an alkaline electrolyte such as potassium hydroxide in water and are generally fuelled with pure hydrogen. The first AFCs operated at between 100ºC and 250ºC but typical operating temperatures are now around 70ºC. Because of the low operating temperature, it is not necessary to employ a platinum catalyst in the system and instead, a variety of non-precious metals can be used as catalysts to speed up the reactions occurring at the anode and cathode. Nickel is the most commonly used catalyst in AFC units.
Due to the rate at which the chemical reactions take place these cells offer relatively high fuel to electricity conversion efficiencies, as high as 60% in some applications. AFCs are the cheapest of fuel cells to manufacture. The catalyst required for the electrodes can be any number of different chemicals that are inexpensive compared to those required for other types of fuel cells.
The commercial prospects for AFCs lie largely with the recently developed bi-polar plate version of this technology, considerably superior in performance to earlier mono-plate versions.
Another recent development is the solid-state alkaline fuel cell, utilizing alkali anion exchange membranes rather than a liquid. This resolves the problem of poisoning and allows the development of alkaline fuel cells capable of running on safer hydrogen-rich carriers such as liquid urea solutions or metal amine complexes.
The world’s first Fuel Cell Ship HYDRA used an AFC system with 5 kW net output.